Electron configuration and orbital diagrams worksheet answers – Unveiling the intricate world of electron configuration and orbital diagrams, this comprehensive worksheet guide unlocks a treasure trove of knowledge, empowering students with the tools to decipher the electronic structures of atoms and unravel the mysteries of chemical bonding.
Through a seamless blend of theoretical explanations and practical examples, this guide illuminates the fundamental concepts of electron configuration, delves into the intricacies of orbital diagrams, and provides a rich collection of solved worksheet questions to reinforce understanding.
Electron Configuration and Orbital Diagrams
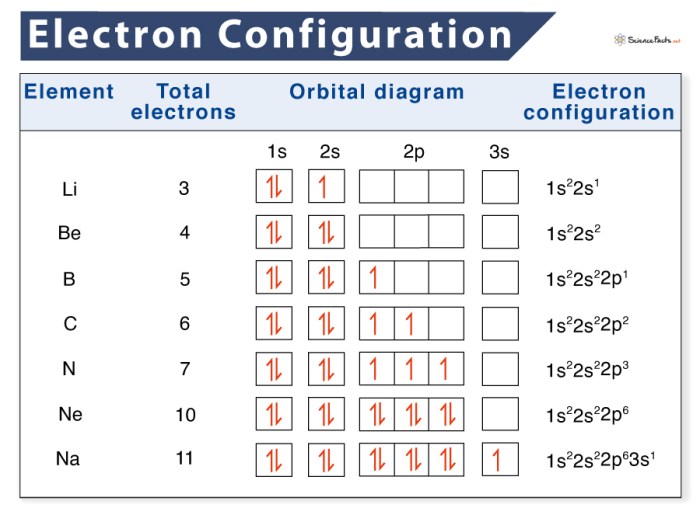
Electron configuration and orbital diagrams are fundamental concepts in chemistry that describe the arrangement and distribution of electrons within an atom. They provide insights into the chemical properties and reactivity of elements.
Electron Configuration
Electron configuration refers to the distribution of electrons in the atomic orbitals of an element. It is typically represented as a string of numbers, where each number represents the number of electrons in a specific energy level (n) and subshell (l).
- The first number represents the energy level (n = 1, 2, 3, …).
- The second number represents the subshell (l = s, p, d, f, …).
- The third number represents the number of electrons in that subshell (2 for s, 6 for p, 10 for d, 14 for f).
For example, the electron configuration of helium (He) is 1s 2, indicating that it has two electrons in the 1s subshell.
Electron configuration follows specific periodic trends, with elements in the same group having similar configurations. For instance, all alkali metals (Group 1) have a single electron in the outermost s subshell.
Orbital Diagrams, Electron configuration and orbital diagrams worksheet answers
Orbital diagrams provide a visual representation of electron configuration. They depict the shape and orientation of the atomic orbitals and indicate the number of electrons occupying each orbital.
- s orbitals are spherical.
- p orbitals are dumbbell-shaped.
- d orbitals have more complex shapes, such as cloverleafs and dumbbells.
- f orbitals have even more complex shapes.
The number of electrons in each orbital is represented by arrows, with each arrow pointing in a different direction to indicate electron spin.
Relationship between Electron Configuration and Orbital Diagrams
Electron configuration and orbital diagrams are closely related. Electron configuration provides the information necessary to construct orbital diagrams, and orbital diagrams provide a visual representation of electron configuration.
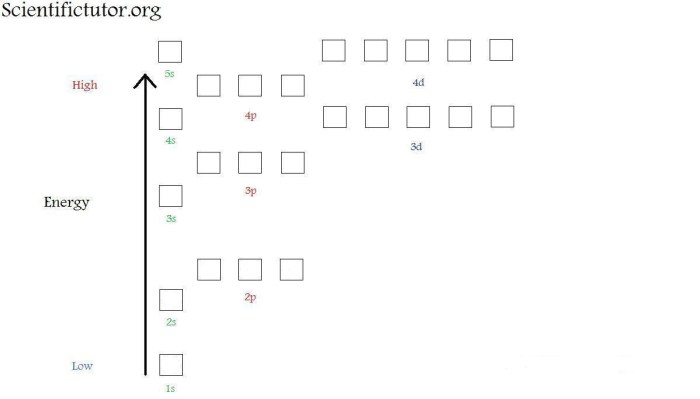
The Aufbau principle states that electrons fill the lowest energy orbitals first, followed by higher energy orbitals. This principle guides the construction of orbital diagrams.
Answers to Common Questions: Electron Configuration And Orbital Diagrams Worksheet Answers
What is electron configuration?
Electron configuration refers to the distribution of electrons in different energy levels or orbitals around the nucleus of an atom.
How do I construct an orbital diagram?
To construct an orbital diagram, first determine the electron configuration of the element. Then, assign the electrons to orbitals based on their energy levels and shapes, following the Aufbau principle and Hund’s rule.
What is the relationship between electron configuration and orbital diagrams?
Electron configuration provides the numerical representation of electron distribution, while orbital diagrams offer a visual representation of the spatial arrangement of electrons in orbitals.