What does the number next to the ions signify – In the realm of chemistry, the numbers accompanying ions hold immense significance, revealing crucial information about their identity and behavior. What does the number next to ions signify? This question delves into the fascinating world of superscript numbers, unlocking their role in understanding oxidation states, charges, and the intricate dance of chemical equations.
Beyond the mere enumeration of atoms, these numbers unveil the underlying structure and properties of ions, enabling chemists to decipher the language of chemical reactions. Embark on a journey of discovery as we explore the profound implications of superscript numbers in chemistry.
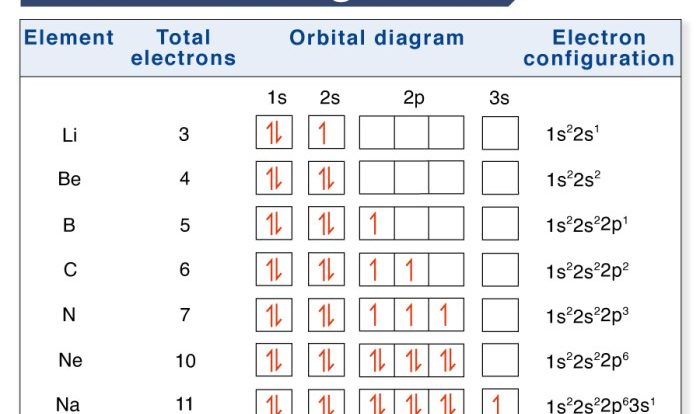
Superscript Numbers
Superscript numbers are small numbers placed above the chemical symbol of an element or ion. They indicate the oxidation state of the element or ion.
For example, in the formula Fe 2+, the superscript number 2+ indicates that the iron ion has a +2 oxidation state. This means that the iron ion has lost two electrons.
Oxidation states are important because they help us to understand the chemical reactions that elements and ions can undergo.
Subscript Numbers

Subscript numbers are small numbers placed below the chemical symbol of an element. They indicate the number of atoms of that element in a molecule or compound.
For example, in the formula H 2O, the subscript number 2 indicates that there are two hydrogen atoms in a water molecule.
Subscript numbers are important because they help us to determine the chemical formula of a compound and to understand its properties.
Charges on Ions

Ions are atoms or molecules that have lost or gained electrons. The number next to an ion indicates its charge.
For example, the ion Na +has a +1 charge because it has lost one electron. The ion Cl –has a -1 charge because it has gained one electron.
The following table summarizes the charges of common ions:
| Ion | Charge |
|---|---|
| H+ | +1 |
| Na+ | +1 |
| K+ | +1 |
| Ca2+ | +2 |
| Mg2+ | +2 |
| Cl– | -1 |
| Br– | -1 |
| I– | -1 |
Balancing Chemical Equations

Chemical equations are used to represent chemical reactions. The numbers next to the ions in a chemical equation indicate the number of moles of each ion that are involved in the reaction.
For example, the following equation represents the reaction between sodium and chlorine to form sodium chloride:
“`
Na + Cl2→ 2 NaCl
“`
The numbers next to the ions indicate that 2 moles of sodium react with 1 mole of chlorine to form 2 moles of sodium chloride.
Balancing chemical equations is important because it ensures that the number of atoms of each element is the same on both sides of the equation.
Essential FAQs: What Does The Number Next To The Ions Signify
What is the significance of superscript numbers in chemistry?
Superscript numbers indicate the oxidation state or charge of an ion, providing insights into its electronic configuration and reactivity.
How do subscript numbers differ from superscript numbers?
Subscript numbers represent the number of atoms of each element in a chemical formula, while superscript numbers signify the charge or oxidation state of an ion.
What is the relationship between the number next to an ion and its charge?
The number next to an ion indicates its charge, with positive numbers denoting cations and negative numbers denoting anions.